Learning Outcomes
i. Students will be able to explain the concept of etherification and describe the preparation of ethers from alcohols.
ii. Students will understand the formation of esters from alcohols and carboxylic acids through esterification reactions.
iii. Students will describe the oxidative cleavage of 1,2-diols using oxidizing agents like sodium periodate (NaIO4) and lead tetraacetate (Pb(OAc)4), leading to the formation of carbonyl compounds.
Introduction
Alcohols, organic compounds with a hydroxyl (-OH) functional group, exhibit a wide range of reactivity, allowing them to undergo various transformations into other functional groups. This lesson delves into the preparation of ethers and esters from alcohols, along with the oxidative cleavage of 1,2-diols, showcasing the versatility of alcohols in organic synthesis.
i. Preparation of Ethers
Ethers are organic compounds characterized by an oxygen atom (-O-) bonded to two alkyl or aryl groups. Alcohols can be converted into ethers through a reaction known as etherification.
Williamson Ether Synthesis: Williamson ether synthesis involves the reaction of an alkoxide ion (generated from an alcohol and a strong base) with an alkyl halide. This method is particularly useful for the preparation of simple ethers.
Acid-Catalyzed Etherification: Alcohols can react with alkenes under acidic conditions to form ethers. This method is often used for the synthesis of cyclic ethers, such as tetrahydrofuran (THF).
ii. Preparation of Esters
Esters are organic compounds characterized by a carbonyl group (C=O) bonded to an alkyl or aryl group and an alkoxy group (-OR). Alcohols can be converted into esters through a reaction known as esterification.
Fischer Esterification: Fischer esterification involves the direct reaction of an alcohol with a carboxylic acid under acidic conditions. This method is a classic approach to ester synthesis, but it often requires prolonged reaction times.
Acid Chloride-Catalyzed Esterification: Alcohols can react with acid chlorides (derivatives of carboxylic acids) in the presence of a base to form esters. This method is more reactive than Fischer esterification and is often used for the synthesis of specific esters.
iii. Oxidative Cleavage of 1,2-Diols
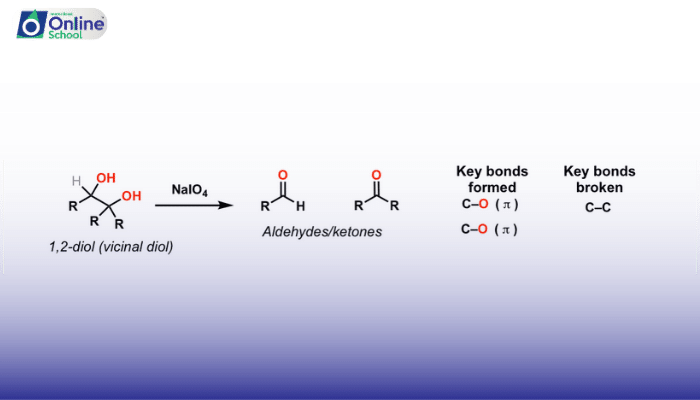
1,2-Diols, compounds containing two hydroxyl groups (-OH) on adjacent carbon atoms, can undergo oxidative cleavage using oxidizing agents to form carbonyl compounds.
Cleavage with Sodium Periodate (NaIO4): NaIO4 selectively cleaves 1,2-diols in cyclic compounds, leading to the formation of vicinal diones (carbonyl groups on adjacent carbon atoms).
Cleavage with Lead Tetraacetate (Pb(OAc)4): Pb(OAc)4 cleaves 1,2-diols in both cyclic and acyclic compounds, resulting in the formation of carbonyl compounds, such as aldehydes, ketones, or dicarbonyls.
Alcohols, with their diverse reactivity, serve as valuable precursors for the synthesis of various organic compounds, including ethers and esters. The preparation of ethers and esters through etherification and esterification reactions, respectively, expands the range of functional groups accessible from alcohols. Additionally, the oxidative cleavage of 1,2-diols using oxidizing agents like NaIO4 and Pb(OAc)4 provides a synthetic route to carbonyl compounds. Understanding the reactivity of alcohols and its applications in etherification, esterification, and oxidative cleavage is crucial for comprehending the versatility of these organic compounds.